Copper Oxide and Sulfuric Acid Formula
Calculate the number of milliequivalents of positive and negative charge respectively. Concentrated nitric acid 68 - 70 is a transparent colorless or yellowish fuming suffocating hygroscopic corrosive liquidThis chemical attacks almost all metals.
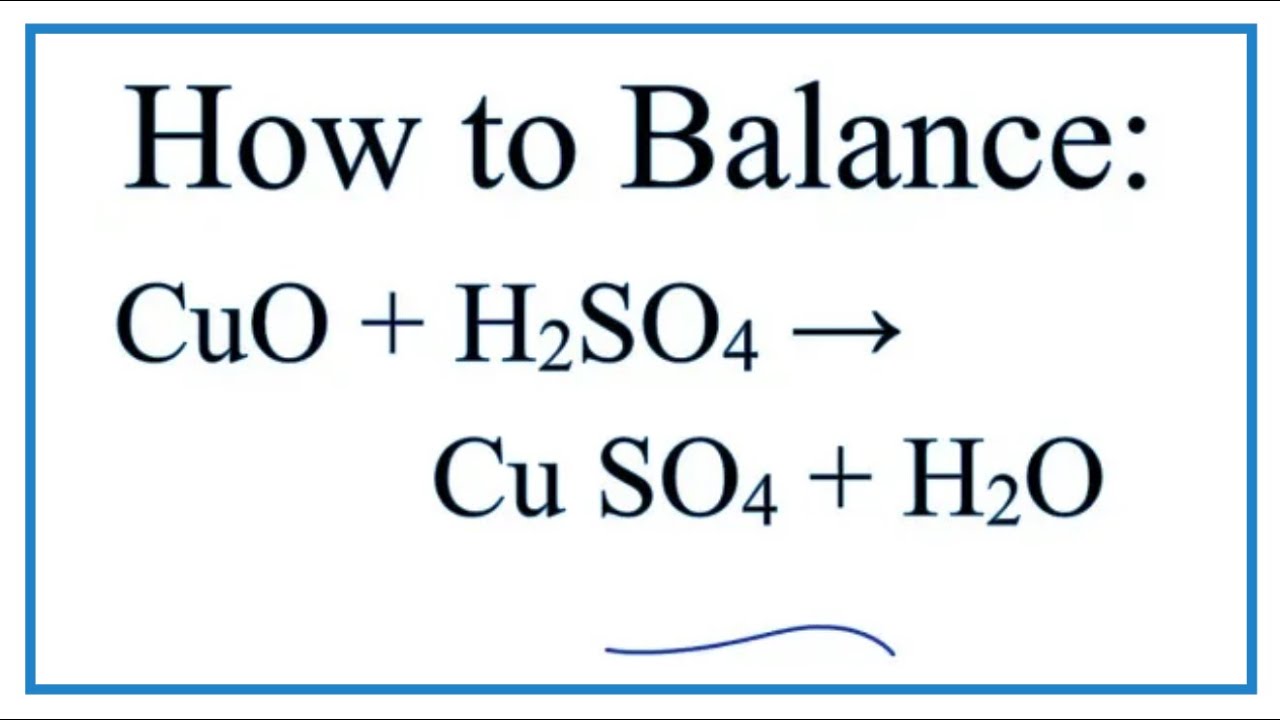
How To Balance Cuo H2so4 Cuso4 H2o Youtube
On top of that this acid is corrosive to metals and most other organic matters like tissue.

. Sulfuric Acid Boiling Point. CopperI oxide or cuprous oxide is the inorganic compound with the formula Cu 2 O. It is approximately 5-20 acetic acid in water.
Sulfuric Acid is a mineral acid with the chemical formula H 2 SO 4. Even materials considered pure elements often develop an oxide coating. It is soluble in water and releases heat on contact.
In a nutshell sulphuric acid is a strong mineral acid characterized by its strong dehydrating and oxidizing nature. For example aluminium. A solution contains the following ions.
Oxide itself is the dianion of oxygen an O 2 molecular ion. For example for H2SO4 each mole of sulfuric acid yields two moles of protons so K 2 and the EW ½ MW. Vinegar is considered a type of weak acid.
It is a colorless to slightly yellow odorless and viscous liquid soluble in water and alcohol used in many applications. Normality K x Molarity----- Sample Exercises. The compound can appear either yellow or red depending on the size of the particles.
It has a strong acidic nature and is corrosive. The molecular formula for water is H 2 O. With oxygen in the oxidation state of 2.
Although it has an extremely low pH value the acetic acid doesnt completely dissociate in water. The structural formula for acetic acid is CH 3 COOH. Ethane-12-diol is reacted with benzene-14-dicarboxylic acid sometimes known as terephthalic acid or its dimethyl ester in the presence of a catalyst to produce initially the monomer and low molecular mass oligomers containing up to about 5 monomer units.
Sulfuric Acid Chemical Properties Hazards And Uses. Sulfuric acid H 2 SO 4 is a strong acid with hygroscopic and oxidizing properties. Sulfuric acid reacts with most metals particularly when diluted with water to form flammable hydrogen gas which may create an explosion hazard.
Registrants and applicants completing the Confidential Statement of Formula CSF Form EPA Form 8570-4 will no longer need to list the commodity inert ingredient suppliers. It is one of the principal oxides of copper the other being or copperII oxide or cupric oxide CuO. Most of the Earths crust consists of oxides.
Buy Nitric Acid Products Online Here Or By Phone. Sulfuric Acid CAS Number. Approximately 50 of the produced sulfuric acid is used in.
It has a chemical formula H 2 SO 4. H2SO4 is an inorganic compound in the acid class. This red-coloured solid is a component of some antifouling paints.
An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Etc per mole of substance. Sulfuric acid is also known as Mattling acid or Oil of vitriol.
Sulfuric acid mono-C12-18-alkyl esters sodium salts. CopperI oxide is found as the reddish. So there are actually two main chemical formulas involved.
Sulfuric-acid 1 5 558 Sulfuric Acid aka. Using the acid provides a direct esterification. This acid is colourless with a pungent smell.
What is Sulfuric Acid. Manufacture of PET a The production of the monomer.
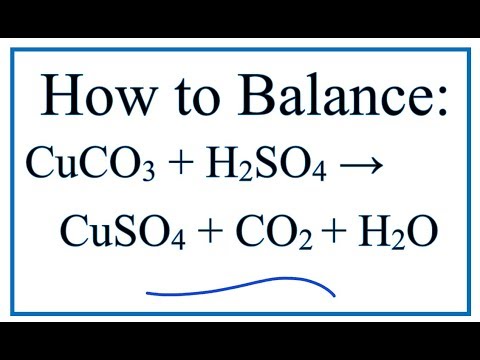
How To Balance Cuco3 H2so4 Cuso4 Co2 H2o Youtube

Pdf Copper Dissolution In Concentrated Sulfuric Acid

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube
No comments for "Copper Oxide and Sulfuric Acid Formula"
Post a Comment